by Stevan Brown, CAFS
What is a desiccant. What are the effects of introducing a desiccant, especially a zeolite, into an air stream of an HVAC system? Will it produce a long term, sustainable benefit in the form of lower humidity in the controlled airspace and can it lower the usage of electricity? What are the effects on odor control? Will the zeolite filter replace the primary particulate filter? What would the economies be?
Desiccants
A range of materials are commonly used to remove moisture from a confined space or from an air stream. These are commonly known as desiccants. A desiccant is a substance with very hygroscopic properties. The essential characteristic of a desiccant is low surface vapor pressure. If the desiccant is cool and dry its surface vapor pressure is low and it can attract moisture from the air which has a high vapor pressure when it is moist. After the desiccant becomes wet and hot its surface vapor pressure is high and it will give off water vapor to the surrounding air. Vapor moves from the air to the desiccant and back again depending on the vapor pressure difference. Desiccants remove moisture in one of three ways; absorption, adsorption or through a chemical reaction or change.
Absorption is when a substance is chemically integrated into another such as salt dissolving in water and becoming salt water. Adsorption is the physical attraction and adherence of gas or liquid molecules to the surface of a solid. The force of the attraction is very small, van der Waal’s forces, and does not change the physical characteristics of the substance. A chemical reaction changes the chemical structure of the substance.
Desiccants are of four types, silica gel, montmorillonite clay, molecular sieve (zeolites), and calcium oxide. Some of these materials can be regenerated (purged of contaminants and reused) and some cannot. It depends on the change in the physical structure of the desiccant and the deleterious effects of regeneration on the material.
When you place a properly prepared desiccant into an air stream with a humidity level higher than that of the desiccant the desiccant will remove moisture up to it’s saturation point or equilibrium capacity. Saturation is when the desiccant is full and even if there are moisture molecules present it will not absorb or adsorb any more moisture. Equilibrium capacity is when the desiccant has pulled so much moisture out of the air that the air retains a stronger hold on the moisture molecules than the desiccant can exert. At equilibrium capacity adding more desiccant will not bring the Relative humidity lower.
Zeolites
Zeolites are a volcanic rock composed of hydrated aluminosilicates of the alkali earth metals. They have three dimensional crystalline frameworks of tetrahedite silica or alumina anions bonded strongly at all corners. Channel sizes of the zeolite structure range from 2.5 to 4.3 angstroms in diameter (according to zeolite type). Specific channel size enables zeolites to act as molecular gas sieves.
Zeolites have a high affinity for water and have the capability of adsorbing and desorbing it without damage to the crystal structure. This property makes them useful as desiccants. When the zeolite is placed in an air stream it will adsorb moisture up to it’s saturation point (as discussed above) and will not, at that point, adsorb any more moisture.
When the level of humidity in the air stream falls below the saturation point of the zeolite it will begin to release moisture back into the air stream. Therefore, it is not “eliminating” moisture, but rather merely holding or releasing it depending on the relative level of humidity of the air stream and the saturation point of the zeolite. To increase the ability of the zeolite to actually remove moisture the desiccant must be regenerated and the extracted moisture must be vented away from the conditioned space. Regeneration of the zeolite is both simple and complex. Approximately 70-80% of the moisture can generally be removed by “pulling a vacuum”, i.e.; lowering the humidity level. This fraction is known as bulk water. The amount of water removed from the zeolite is dependent on how low the humidity is lowered but, without a method to exhaust this extracted water, the moisture merely leaves the zeolite and re-enters the air stream. This again lowers the saturation point of the zeolite. Therefore, the zeolite adsorbs moisture until it cannot adsorb more. If the humidity level falls the zeolite deadsorbs moisture until it is in “balance” with the lower humidity level. At that point it begins to adsorb moisture and the cycle is repeated.
The remaining water (which cannot be removed by pulling a vacuum) is very difficult to remove and may require prolonged heating at moderate (150C or 212F, the boiling point of water) to very high (600-800C, 1112-1472F) temperatures. This fraction is coordinated to the metal cations present in the zeolite. There would need to be a chemical analysis of the particular zeolite (s) in use to determine the characteristics before it could be determined what level of heat is required to sufficiently remove lower levels of moisture. It should also be noted that heating zeolites to very high temperatures can cause extensive structural damage.
Zeolites are commonly used in dehumidification devices known generally as desiccant wheels. These devices lower humidity levels and provide many benefits over the sustainable long term. However, it is very important to realize that these devices continually regenerate the zeolite or other desiccant by passing the saturated desiccant through a heated air stream and venting the moisture laden exhaust away from the air stream and conditioned space. This is necessary to prevent the captured moisture from being reintroduced into the conditioned space. In practice, this is the only way zeolites are used to control humidity levels in conditioned spaces. The zeolite adsorbs water at or near to the saturation point and then is rotated out of the air stream and into a heated exhaust stream. This regenerates the zeolite on a continuing basis, exhausts the moisture away from the conditioned space, and effectively eliminates moisture from the area being treated. While this is a fairly simple description of the desiccant wheels they are actually quite complex and expensive (a drawing below illustrates a desiccant wheel system). There is no way to eliminate the moisture from the conditioned space with zeolites without continual regeneration and an exhaust mechanism.
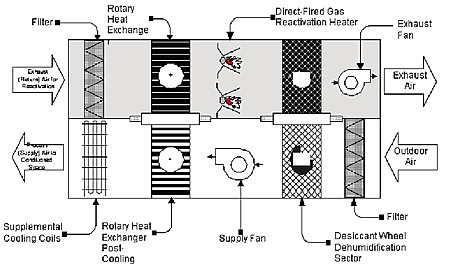
Fig. 1. Schematic of the Two-Wheel Desiccant System
It is also important to note that zeolites cannot, by themselves break water into hydrogen and oxygen. This would entail a chemical change and as we have already discussed zeolites work through adsorption (again, van der Waal’s forces). You could incorporate other molecules into the zeolites which could accomplish this but the zeolite would merely be acting as a media host (like a sponge) to allow the reaction when the various chemicals (a sensitizer, an electron relay and a sacrificial donor/acceptor) are introduced to achieve splitting. To date there is not an economical or feasible way to produce this phenomena on a prolonged basis nor could you supply the various chemicals needed to continue this process without some other mechanism being installed. Zeolites, because they are molecular sieves, can be very effective at separating compounds in an air, gas or, water or chemical stream but do not split molecules. For example, if you want to remove a particular gas molecule from an industrial process stream you would determine the appropriate zeolite by the channel size needed. You would then pass the process stream through the zeolite. Because the channel size of the zeolite would have been selected to trap the particular gas molecule you could separate these gas molecules from the process stream. But it would not break the gas molecule into it’s constituent atoms.
Hydrogen
To demonstrate that zeolites do not split water molecules into hydrogen and oxygen it is useful to look at current hydrogen production methods. Hydrogen production in the U. S. amounts to about 3 billion cubic feet per year. There are currently five methods used to produce hydrogen; steam on heated carbon, decomposition of certain hydrocarbons with heat, reaction of sodium or potassium hydroxide on aluminum, electrolysis of water, or displacement from acids by certain metals. It should be noted that none of these processes utilize zeolites as the agent to effect a chemical change or splitting. One of the major impediments currently to fuel cell development is a reliable, affordable way to obtain hydrogen without producing other pollutants. Keep in mind that, even though hydrogen is the most abundant matter in the universe it does not exist by itself (on Earth anyway) except in trace amounts in the atmosphere. We cannot merely pump it from air.
Also, hydrogen is an extremely flammable gas. It seems unlikely, even if zeolites could break out hydrogen, that you would want to introduce pure hydrogen into an air stream and then pass that air stream over electrical equipment. It should also be noted that hydrogen combines with other elements to form very noxious (hydrogen sulfide) and/or very toxic and corrosive (hydrogen chloride) compounds.
Odor Control and Removal
In general, zeolites are much less effective than carbon for odor control. While zeolites perform almost as well as carbon for ammonia odors they are inferior in virtually all other instances and have little or no ability to eliminate many odors. Zeolites, like carbon, can be used in conjunction with other reactive agents, such as potassium permanganate, to remove some specific contaminants. It is not the zeolite that is effective however, it is the additional agent. Also, to regenerate zeolites requires the same mechanisms as discussed in moisture regeneration and it is unlikely that low temperatures would be effective. Further, it would not be wise to bake these zeolites in indoor ovens because you would not know what contaminants might be released into the indoor space. In practice, zeolites are not regenerated except in mechanisms like desiccant wheels which exhaust to the outdoors. In contrast, activated carbon has a capacity for virtually all vapor contaminants. This is why, activated carbon or impregnated carbon are used almost exclusively in vapor contaminant control and removal.
Price of Zeolites
Zeolites are inexpensive, readily available and can be easily purchased. The Zeolite Clinitilophite is available at about $200 a ton or 10 to 14 cents a pound. Chabazite is slightly more.